Cooling Curves For A Liquid Solid System

Cooling Curves For A Liquid Solid System Youtube 12.5: interpretation of cooling curves. page id. dissemination of it for the promotion of materials science (doitpoms) university of cambridge. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. A cooling curve for a sample that begins at the temperature and composition given by point a is shown in figure 8.10.1b 8.10. 1 b. figure 8.10.1 8.10. 1: (a) cooling of a two component system from liquid to solid. (b) cooresponding cooling curve for this process. as the sample cools from point a, the temperature will decrease at a rate.
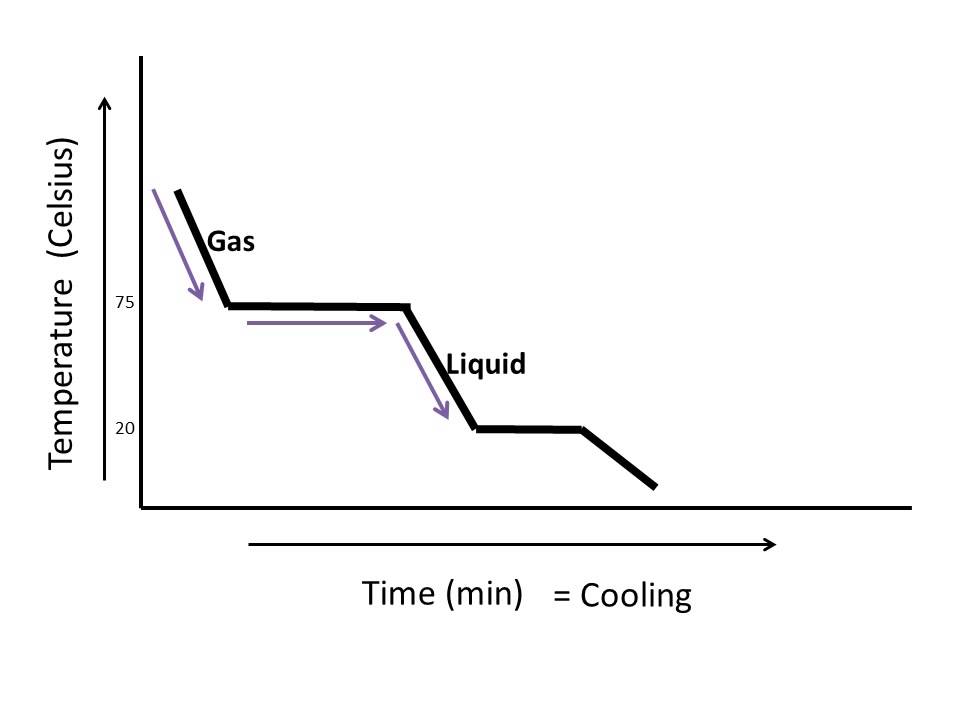
How To Read A Cooling Curve Youtube Organized by textbook: learncheme uses the information in a phase diagram to draw the temperature dependence on time as a binary liquid alloy is. Cooling curves for tin lead mixtures. if you add some tin to the lead, the shape of the cooling curve changes. the next graph shows what happens if you cool a liquid mixture containing about 67% lead and 33% tin by mass. there are lots of things to look at: notice that nothing happens at all at the normal freezing point of the lead. Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is. The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant.

Heating And Cooling Curves Overview Examples Expii Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is. The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. Cooling curves. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. the gradient of the cooling curve is related to the heat capacity, the thermal conductivity of the substance, and the external temperature. the more heat is required to change the temperature of the substance, the. For a lot of systems, the liquid curve will overlap with the upturned section (between the two minima) of the solid free energy curve for a range of temperatures. at one specific temperature a common tangent can be drawn for the liquid and solid curves. it is tangent to the solid curve in two places and to the liquid curve in one place.

Digging Into Phase Diagrams Cooling Curves Physical Chemistry Cooling curves. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. the gradient of the cooling curve is related to the heat capacity, the thermal conductivity of the substance, and the external temperature. the more heat is required to change the temperature of the substance, the. For a lot of systems, the liquid curve will overlap with the upturned section (between the two minima) of the solid free energy curve for a range of temperatures. at one specific temperature a common tangent can be drawn for the liquid and solid curves. it is tangent to the solid curve in two places and to the liquid curve in one place.

Comments are closed.