Fda Warms 8 Companies For Unapproved Eye Items Obn

Fda Warns Cvs Health Walgreens Against Unapproved Eye Products Jeremy kahn. (301) 796 8671. consumer: 888 info fda. fda has issued warning letters to eight companies for manufacturing or marketing unapproved ophthalmic drug products in violation of federal law. Fda warns 8 companies for marketing unapproved eye products. september 13, 2023. on monday, the us food and drug administration (fda) took action by sending warning letters to several companies, including cvs and walgreens, due to concerns regarding the manufacturing and marketing of unapproved eye products. according to the fda, these products.

Fda Issues Warning Letters To 8 Companies For Marketing Unapproved Eye Products In warning letters, fda tells 8 companies to stop selling illicit eye drugs by fraiser kansteiner sep 12, 2023 3:47pm u.s. fda warning letter ophthalmology unapproved drugs. Per the fda, the companies’ unapproved eye products “are illegally marketed to treat conditions such as conjunctivitis (“pink eye”), cataracts, glaucoma and others.”. the agency also stated that a few of the letters include quality issues related to product sterility for some of the companies. The fda has issued warning letters to 8 companies for manufacturing or marketing unapproved ophthalmic drug products in violation of federal law. these warnings come amidst an increased amount of complications and issues caused by unapproved eye drops , as the fda states they are trying to “protect americans from potentially harmful. The us food and drug administration on monday sent warning letters to cvs, walgreens and other companies over manufacturing and marketing of unapproved eye products the agency says could pose a.

Fda Warns Companies Selling Unapproved Eye Products Medtruth Prescription Drug Medical The fda has issued warning letters to 8 companies for manufacturing or marketing unapproved ophthalmic drug products in violation of federal law. these warnings come amidst an increased amount of complications and issues caused by unapproved eye drops , as the fda states they are trying to “protect americans from potentially harmful. The us food and drug administration on monday sent warning letters to cvs, walgreens and other companies over manufacturing and marketing of unapproved eye products the agency says could pose a. Fda warns walgreens, cvs, others over unapproved eye products. fda sent warning letters to 8 companies concerning unapproved eye products. unapproved eye products pose a heightened risk of harm to users, fda says. cvs said in a statement it has since stopped selling the items. updated: sep 14, 2023 08:24 am cdt. The fda has issued warning letters to eight companies for manufacturing or marketing unapproved ophthalmic drug products, the agency announced in a press release.in an effort to protect u.s.
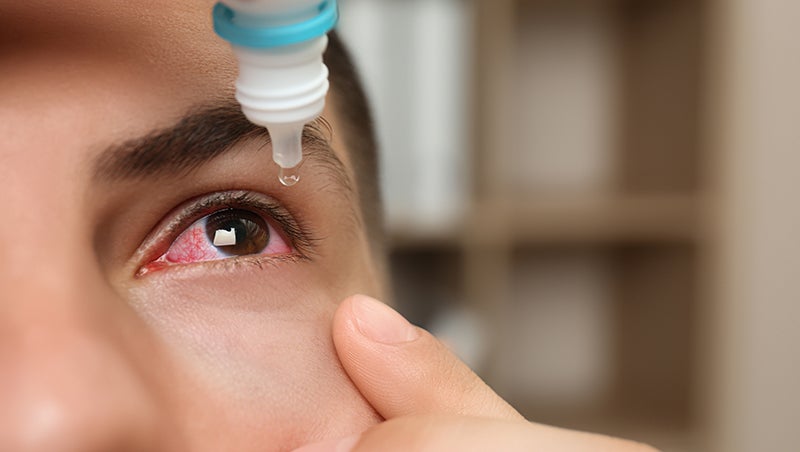
Fda Warns Eight Companies Regarding Unapproved Ophthalmic Drugs The Coastland Times The Fda warns walgreens, cvs, others over unapproved eye products. fda sent warning letters to 8 companies concerning unapproved eye products. unapproved eye products pose a heightened risk of harm to users, fda says. cvs said in a statement it has since stopped selling the items. updated: sep 14, 2023 08:24 am cdt. The fda has issued warning letters to eight companies for manufacturing or marketing unapproved ophthalmic drug products, the agency announced in a press release.in an effort to protect u.s.

Fda Warns Companies About Unapproved Ophthalmic Products Medpage Today

Comments are closed.