Learn All About The Strong Acids And Bases Praxilabs
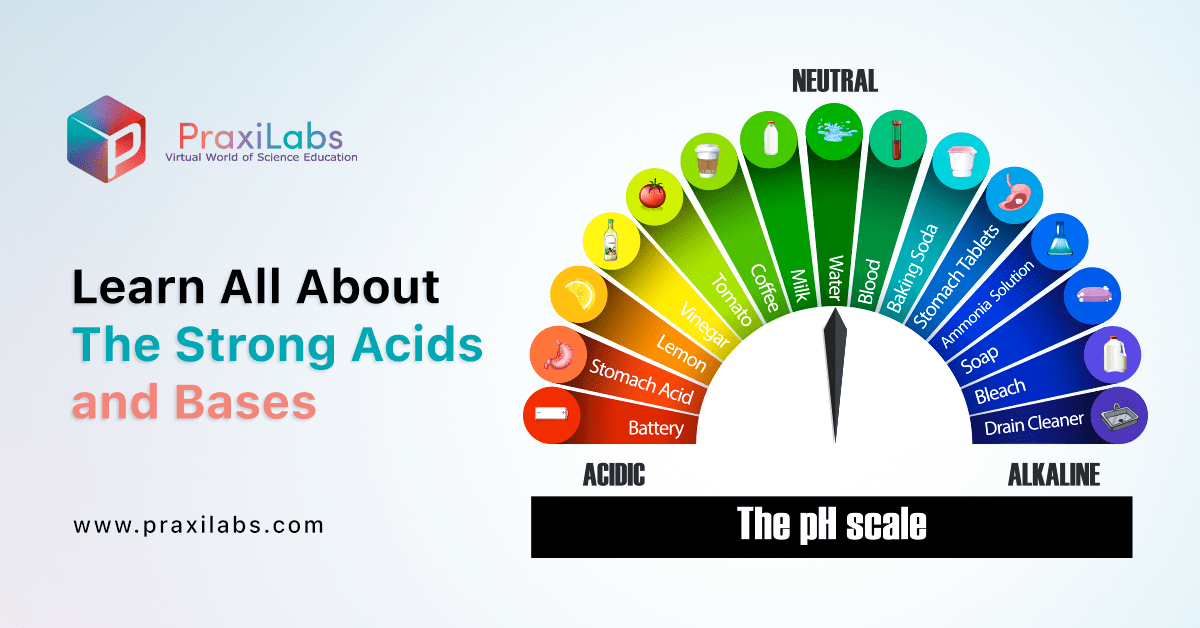
Learn All About The Strong Acids And Bases Praxilabs The more h ions, the more the acidity of it and the lower the ph value will be. bases have a ph value above 7. when there is a balance of h and oh– ions, it is called neutral and ph value is 7. for very strong acids, the ph value can be less than zero and for very strong bases, the ph value can be more than 14. Learning objectives (ilo’s) <p>gain the knowledge of how acids and bases will react if their formulas are known. understand the titration concept. standardize an aqueous solution of sodium hydroxide to be used as the titrant. calculate the concentration of an unknown strong acid given the amount of base necessary to titrate it.
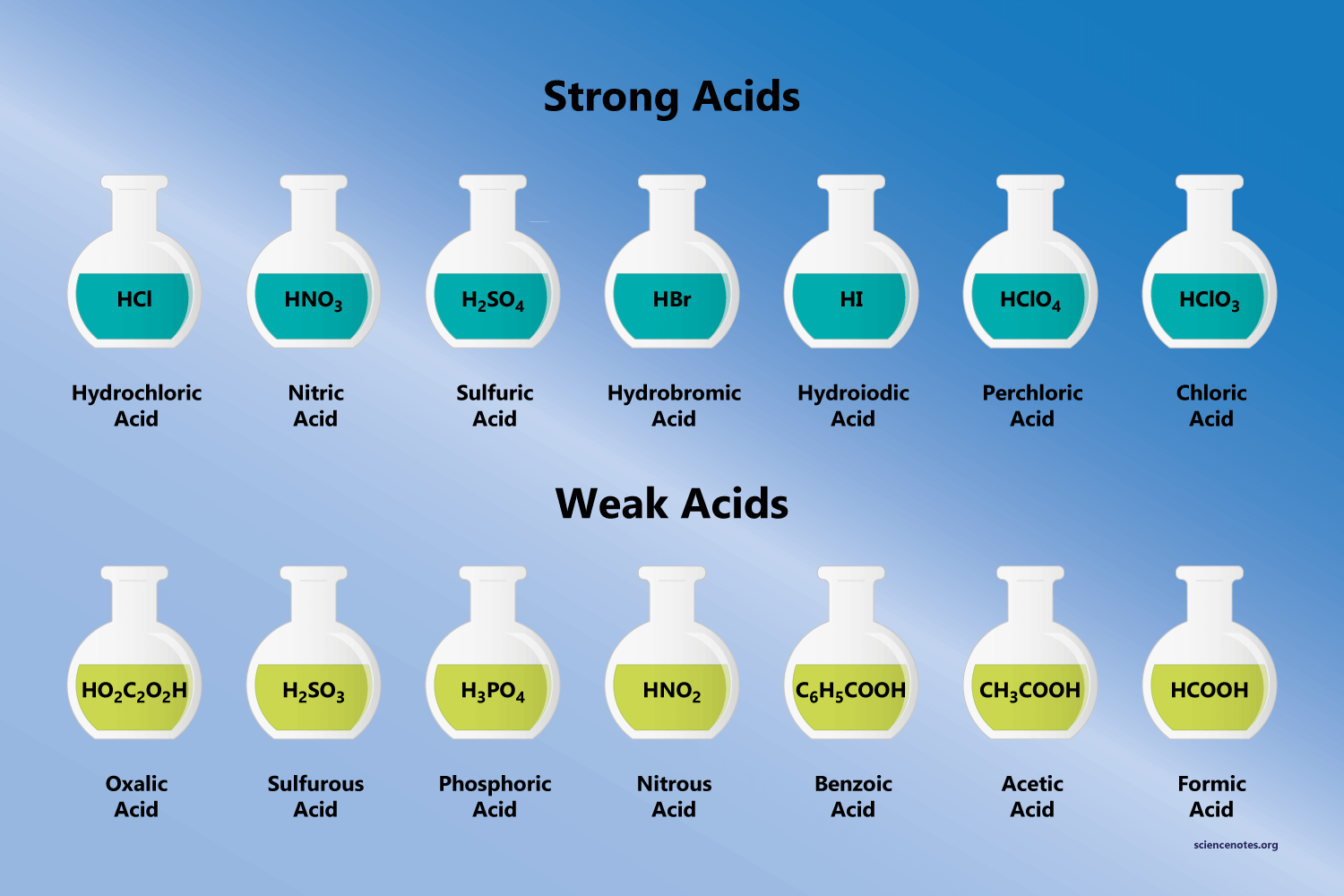
Learn All About The Strong Acids And Bases Praxilabs Sr (oh)2. strontium hydroxide (strong base electrolyte) ba (oh)2. barium hydroxide (strong base electrolyte) nh3 () ammonia (weak base electrolyte) nr3 (nch3h2, n (ch3)3) amines (weak base electrolyte) strong acids bases= strong electrolyte, ionizes completely in water; weak acids bases (not comprehensive) = weak electrolyte, partial ionization. Acids and bases are also sub classified into weak and strong acids and bases, depending on the strength. 📋 in this article we will focus on strong acids and bases but first we will clarify some. Ha(aq) h 2o(l) → h 3o (aq) a − (aq) water is the base that reacts with the acid ha, a − is the conjugate base of the acid ha, and the hydronium ion is the conjugate acid of water. by definition, a strong acid yields 100% of h 3o and a − when the acid ionizes in water. table 16.4.1 lists several strong acids. table 16.4.1 16.4. 1. Study with quizlet and memorize flashcards containing terms like strong acids: *no* *so*uls *br*ing *i*diots *clo*wny *clo*tted *cl*ams, strong bases: *li*ly and *na*te *r*o*b*bed a *ba*nk and *k*ept a *c*razy *s*tash of *ca*sh, weak acids: *so* *po*pcorn *f*orces me to drink *ch*o*co*late milk *co*vered with *po*sicles and more.
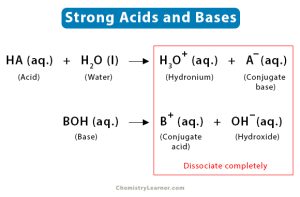
Learn All About The Strong Acids And Bases Praxilabs Ha(aq) h 2o(l) → h 3o (aq) a − (aq) water is the base that reacts with the acid ha, a − is the conjugate base of the acid ha, and the hydronium ion is the conjugate acid of water. by definition, a strong acid yields 100% of h 3o and a − when the acid ionizes in water. table 16.4.1 lists several strong acids. table 16.4.1 16.4. 1. Study with quizlet and memorize flashcards containing terms like strong acids: *no* *so*uls *br*ing *i*diots *clo*wny *clo*tted *cl*ams, strong bases: *li*ly and *na*te *r*o*b*bed a *ba*nk and *k*ept a *c*razy *s*tash of *ca*sh, weak acids: *so* *po*pcorn *f*orces me to drink *ch*o*co*late milk *co*vered with *po*sicles and more. The strongest acids are at the bottom left, and the strongest bases are at the top right. the conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. strong acids are h3o plus, hno3, h2so4, hcl, and hbr. negligible acids are hs minus and oh minus. stron bases are o negative. This is a list of the strong acids and strong bases. there aren’t very many, so it’s a good idea to memorize them, if you can. table of strong acids the strong acids ionize completely in water to yield or or more protons per acid molecule. name formula ionization hydrogen iodide or hydroiodic acid hi h (aq) […].
Learn All About The Strong Acids And Bases Praxilabs The strongest acids are at the bottom left, and the strongest bases are at the top right. the conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. strong acids are h3o plus, hno3, h2so4, hcl, and hbr. negligible acids are hs minus and oh minus. stron bases are o negative. This is a list of the strong acids and strong bases. there aren’t very many, so it’s a good idea to memorize them, if you can. table of strong acids the strong acids ionize completely in water to yield or or more protons per acid molecule. name formula ionization hydrogen iodide or hydroiodic acid hi h (aq) […].

Comments are closed.