Phase Diagrams And Heating Cooling Curves

Heating And Cooling Curves Read Chemistry Ck 12 Foundation Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific. The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant.

Label Each Region Of The Cooling Curve Hadley Has Hendrix Heating and cooling curves are graphs. they plot a substance's temperature (y axis) against heat (x axis). for heating curves, we start with a solid and add heat energy. for cooling curves, we start with the gas phase and remove heat energy. cooling and heating curves have five segments. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have. Key concepts and summary. phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). these two types of plots provide complementary information on the phase transitions of substances. while a heating curve provides information on the phase changes at a single pressure, the phase. Phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). these two types of plots provide complementary information on the phase transitions of substances. while a heating curve provides information on the phase changes at a single pressure, the phase diagram depicts the phase.
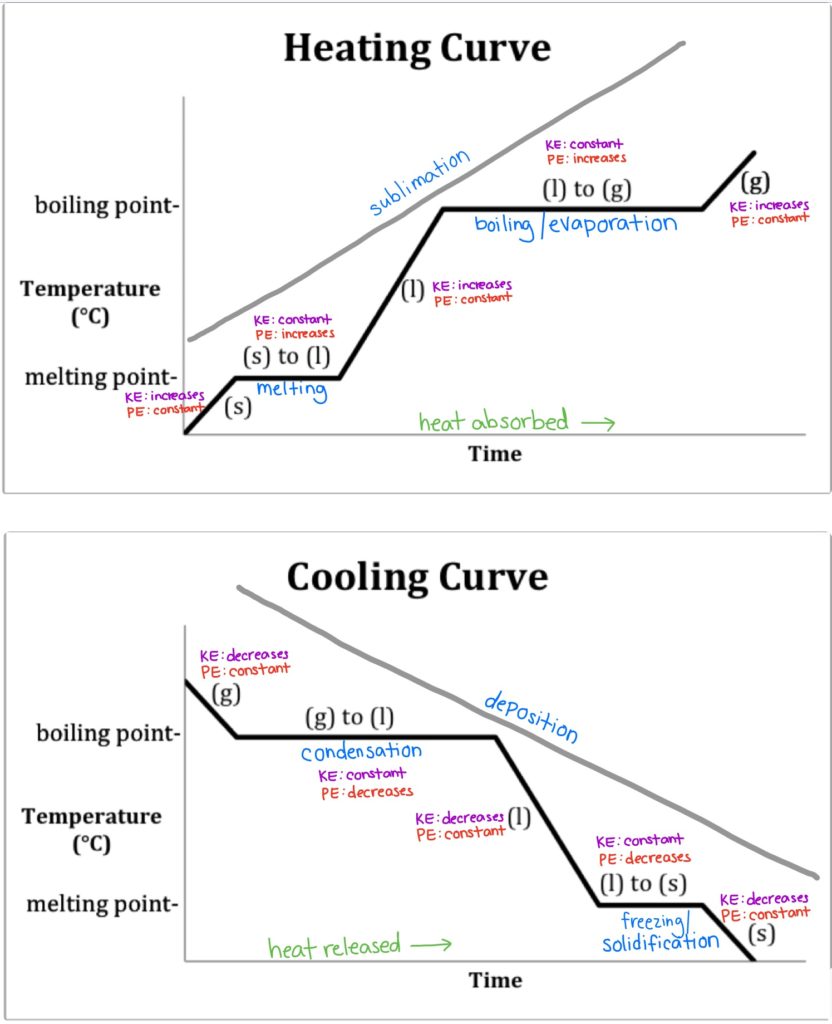
Heating And Cooling Curves Key concepts and summary. phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). these two types of plots provide complementary information on the phase transitions of substances. while a heating curve provides information on the phase changes at a single pressure, the phase. Phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). these two types of plots provide complementary information on the phase transitions of substances. while a heating curve provides information on the phase changes at a single pressure, the phase diagram depicts the phase. Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down.

Comments are closed.