The Complete Guide To Understanding Cooling Curve Phase Diagrams

The Complete Guide To Understanding Cooling Curve Phase Diagrams The cooling curve phase diagram is a graphical representation of this solidification process, which helps us understand the behavior of different substances during solidification. the cooling curve phase diagram consists of two axes: temperature and time. it shows the temperature of the substance on the y axis and the time on the x axis. By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa.

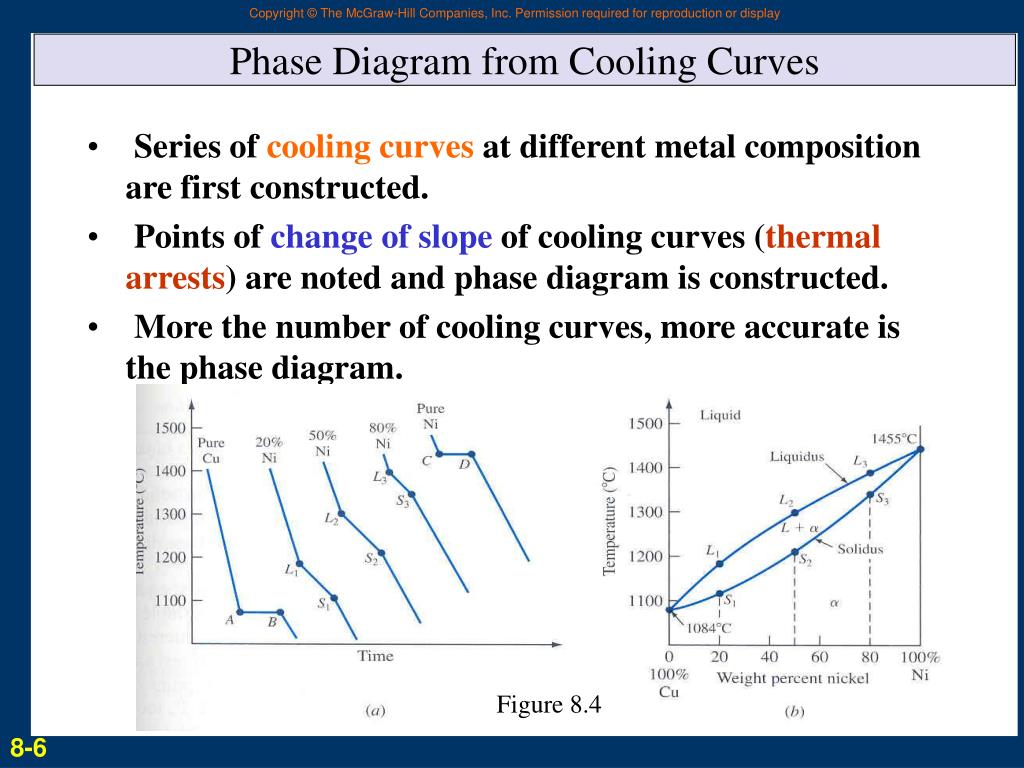
The Complete Guide To Understanding Cooling Curve Phase Diagrams Interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point is. A cooling curve for a sample that begins at the temperature and composition given by point a is shown in figure 8.10.1b 8.10. 1 b. figure 8.10.1 8.10. 1: (a) cooling of a two component system from liquid to solid. (b) cooresponding cooling curve for this process. as the sample cools from point a, the temperature will decrease at a rate. Interpretation of cooling curves. the free energy curves and phase diagrams discussed in phase diagrams 1 were all for systems where the solid exists as a solution at all compositions and temperatures. in most real systems this is not the case. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling.

The Complete Guide To Understanding Cooling Curve Phase Diagrams Interpretation of cooling curves. the free energy curves and phase diagrams discussed in phase diagrams 1 were all for systems where the solid exists as a solution at all compositions and temperatures. in most real systems this is not the case. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling. Figure 16.3.1 16.3. 1: a heating curve for water. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: a–b: heating solid ice; b–c: melting ice; c–d: heating liquid water; d–e: vaporizing water; e–f: heating steam. thus the temperature of a system does. 242 phase diagrams—understanding the basics ing curves shown in fig. 12.3, where the effects of both supercooling and superheating can be seen. the dip in the cooling curve often found at the start of freezing is caused by a delay in the start of crystallization. the continual freezing that occurs during cooling through a two phase.

Digging Into Phase Diagrams Cooling Curves Physical Chemistry Figure 16.3.1 16.3. 1: a heating curve for water. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: a–b: heating solid ice; b–c: melting ice; c–d: heating liquid water; d–e: vaporizing water; e–f: heating steam. thus the temperature of a system does. 242 phase diagrams—understanding the basics ing curves shown in fig. 12.3, where the effects of both supercooling and superheating can be seen. the dip in the cooling curve often found at the start of freezing is caused by a delay in the start of crystallization. the continual freezing that occurs during cooling through a two phase.
Cooling Curve Phase Diagram

Comments are closed.