Thermal Conduction Explanation And Example

Thermal Conduction Explanation For Fourier S Law Stock Illustration Illustration Of The units of heat transfer are the joule (j), calorie (cal), and kilocalorie (kcal). the unit for the rate of heat transfer is the kilowatt (kw). the three types of heat transfer with examples. the three types of heat transfer differ according to the nature of the medium that transmits heat: conduction requires contact. convection requires. For example, the thermal conductivities of silver and copper are 406 wm 1 k 1 and 385 wm 1 k 1, respectively. insulators are poor conductors of heat. they have voids in between the atoms, which interfere with heat transfer. for example, the thermal conductivity of wood ranges from 0.04 to 0.12 wm 1 k 1. air is a poor conductor of heat. its.
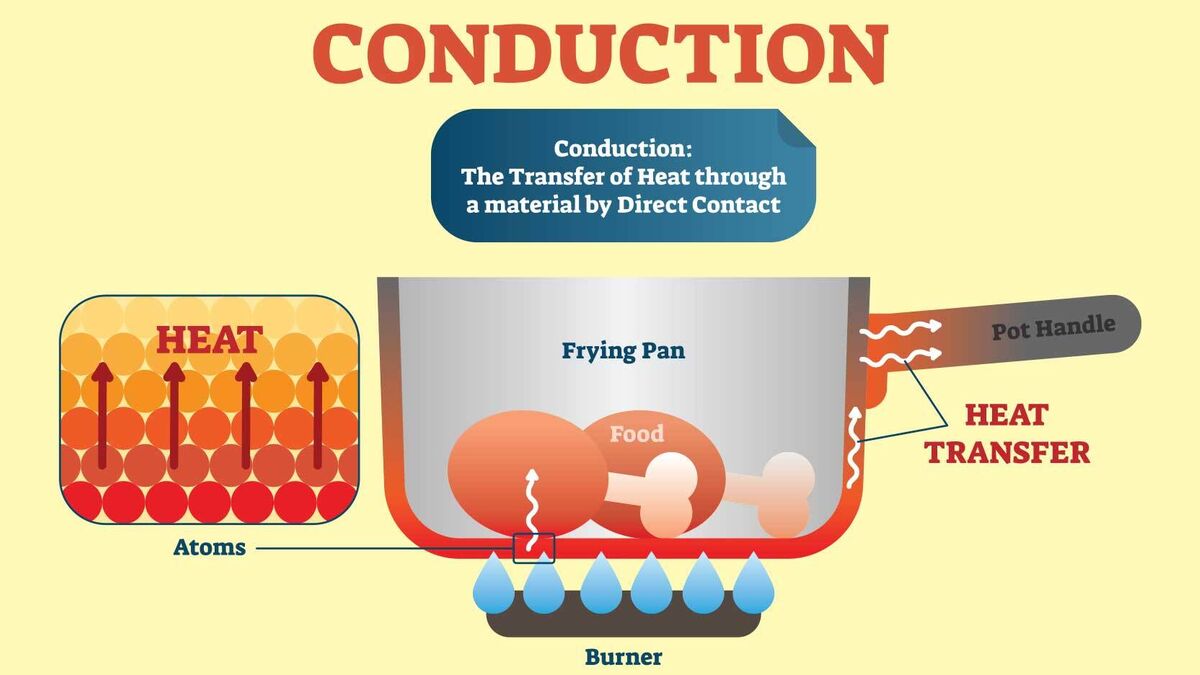
Examples Of Heat Energy There are three forms of thermal energy transfer: conduction, convection, and radiation. conduction involves molecules transferring kinetic energy to one another through collisions. convection occurs when hot air rises, allowing cooler air to come in and be heated. thermal radiation happens when accelerated charged particles release electromagnetic radiation, which can be felt as heat. Thermal conduction (power) is the heat per unit time transferred some distance ℓ between the two temperatures. κ is the thermal conductivity of the material. a is the cross sectional area of the object. Δt is the difference in temperature from one side to the other. ℓ is the length of the path the heat has to be transferred. 1. conduction. heat is transferred between two atoms or molecules in direct contact. the transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures, resulting in collisions. heat transfer occurs through vibrations if the atoms are fixed in a lattice. conduction takes place in solid, liquid, and gas. Example 13.4.1 13.4. 1: calculating heat transfer by convection: convection of air through the walls of a house. most houses are not airtight: air goes in and out around doors and windows, through cracks and crevices, following wiring to switches and outlets, and so on. the air in a typical house is completely replaced in less than an hour.
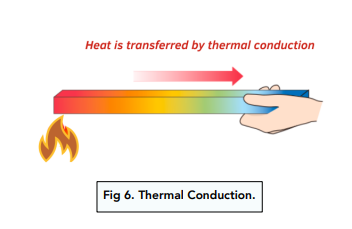
Thermal Conduction Gcse Physics Study Mind 1. conduction. heat is transferred between two atoms or molecules in direct contact. the transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures, resulting in collisions. heat transfer occurs through vibrations if the atoms are fixed in a lattice. conduction takes place in solid, liquid, and gas. Example 13.4.1 13.4. 1: calculating heat transfer by convection: convection of air through the walls of a house. most houses are not airtight: air goes in and out around doors and windows, through cracks and crevices, following wiring to switches and outlets, and so on. the air in a typical house is completely replaced in less than an hour. Summary. heat conduction is the transfer of heat between two objects in direct contact with each other. the rate of heat transfer q t (energy per unit time) is proportional to the temperature difference t2 − t1 and the contact area a and inversely proportional to the distance between the objects: q t = ka(t2 − t1) d. Conduction heat transfer examples: 1] for the wall shown below, the area perpendicular to the direction of flow is 1.5 m². the temperature at the left side of the wall is 1500°c and at the right side is 40°c. thus find the rate of heat transfer through the wall.

Thermal Conduction Teaching Resources Summary. heat conduction is the transfer of heat between two objects in direct contact with each other. the rate of heat transfer q t (energy per unit time) is proportional to the temperature difference t2 − t1 and the contact area a and inversely proportional to the distance between the objects: q t = ka(t2 − t1) d. Conduction heat transfer examples: 1] for the wall shown below, the area perpendicular to the direction of flow is 1.5 m². the temperature at the left side of the wall is 1500°c and at the right side is 40°c. thus find the rate of heat transfer through the wall.

Comments are closed.